CHEMISTRY NOTES
QUANTUM MECHANICS
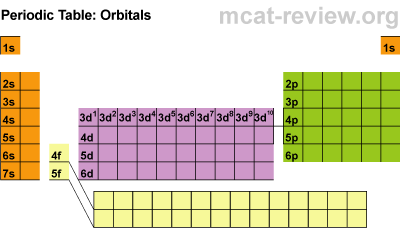
· Different levels of electrons: s, p, d, and f orbitals
· As you increase levels, the orbitals get larger
BOHRS THEORY:
· The electron is a particle that must be in orbital in the atom
QUANTUM THEORY:
· The electron is like a cloud of negative energy or a wave
· Orbitals are areas in 3D spaces where the electrons most probably are
· The energy of the electron is in its vibrational modes – like notes on a guitar string
· Photons are produced when high energy modes change to lower energy modes
S ORBITALS:
· Each orbital holds 2 electrons
(hydrogen and helium)
P ORBITALS
· There are 3 suborbitals
· Each contains 2 electrons
· Total of 6 electrons (3 x 2 = 6)
D ORBITALS
· There are 5 suborbitals
· Each contains 2 electrons
· Total electrons: (2 x 5 = 10)
F ORBITALS
· There are 7 suborbitals
· Each contains 2 electrons
· Total electrons: (7 x 2 = 14)
· Can be made into nuclear bombs
Examples:
1. How many and what type of electrons does the atom calcium have?
1s2 2s2 2p6 3s2 3p6 4s2
No comments:
Post a Comment