ORGANIC CHEMISTRY - ALKANES
- Organic chemistry is the study of carbon compounds
- Carbon will form multiple covalent bonds
- Carbon compounds can form chain rings or branches
- There are less than 100000 non organic compounds
- Organic compounds number more than 17 000 000
- The simplest organic compounds are made of Carbon and hydrogen
When naming an organic compound take the longest chain of carbon that are connected.
C - C - C - C - C 5 carbon chain - PENTANE
\
Use prefixes to name the carbon chains:
1 - meth
2 - eth
3- pro
4 - but
5 - pent
6 - hex
7 - hept
8 - oct
9 - non
10 - dec
when dealing with Alkanes the ending will always be a preffix + ane. (ie. propane, decane, methane etc)
EXAMPLES!
Here are some very basic alkanes, can you name them?
C - C = Ethane
C - C - C - C = Butane
C = Methane
Now as mentioned before, organic chemistry deals with Carbons AND HYDROGENS. So whenever you see an open valence electron on a carbon, pair it with a hyrdrogen. (Carbon can make 4 bonds)
EXAMPLES!
Methane - CH(4)
Butane - CH(3) - CH(2) - CH(2) - CH(4)
Now moving on to the final piece of information dealing with organic compounds. When a compound has a normal chain, with several smaller ones sticking out of it, you name the side chain with the same prefixes but then add the ending - yl. Also you number the chains from left to right, and put the number of the side chain next to the prefix, AND, your side chains have to add up to be the lowest possible number.
EXAMPLES!
C - C - C - C - C 2 methyl pentane
C
Also, when there is multiple chains of the same number use prefixes such as:
di
tri
tetra
etc
Examples:
C - C - C -C 2, 3 dimethyl butane
C C
C - C - C - C -C 2 ethyl pentane
C
C
 propyl methyl ether
propyl methyl ether dimethyl ether
dimethyl ether
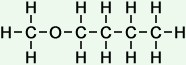
 (butanoic, or butyric acid)
(butanoic, or butyric acid)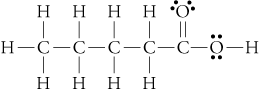

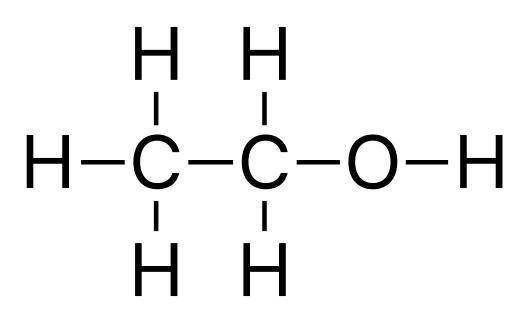 Ethanol
Ethanol Ethanediol
Ethanediol
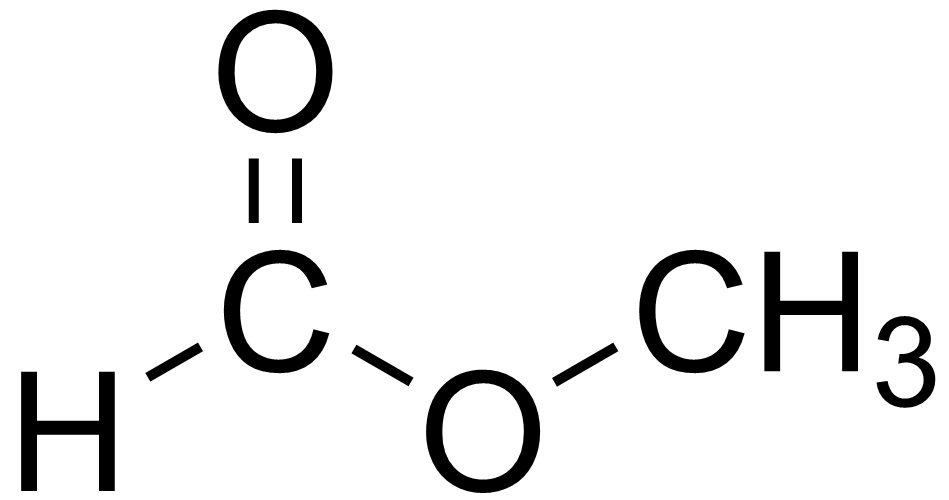
 methyl ethanoate
methyl ethanoate ethyl ethanoate
ethyl ethanoate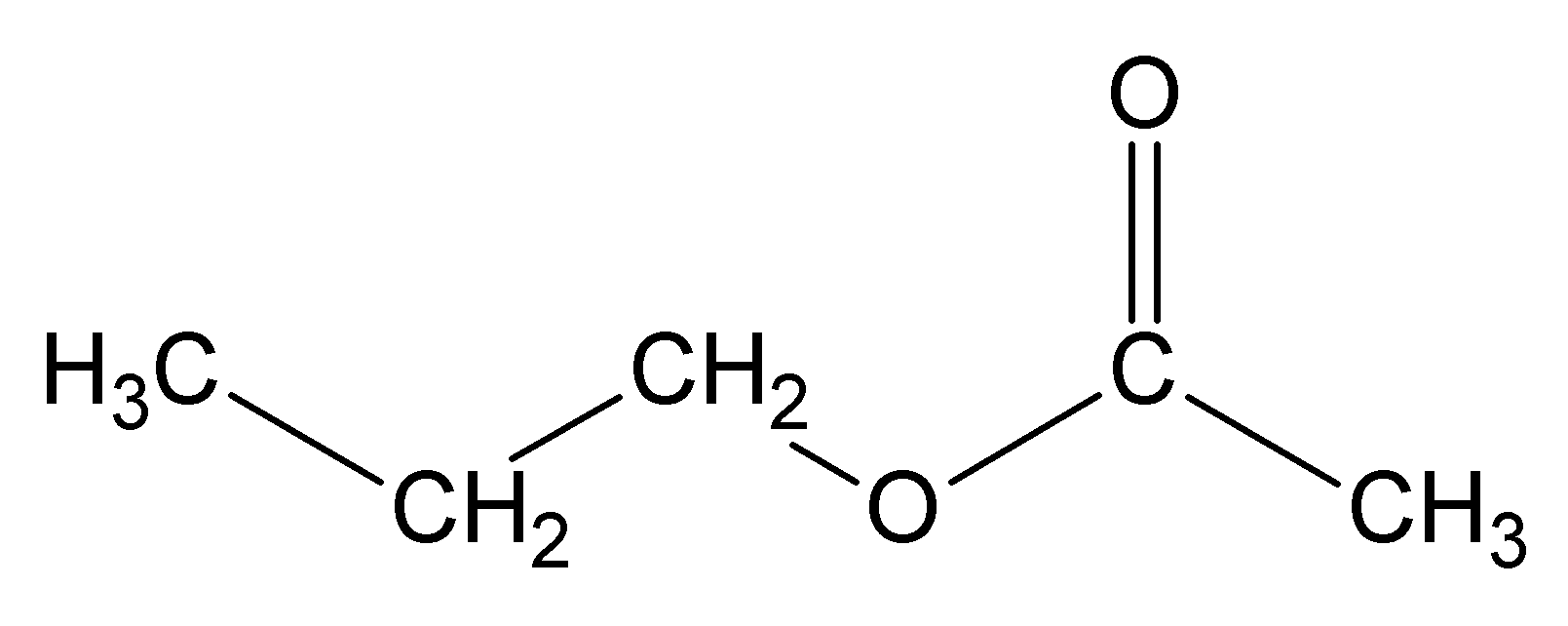
 \
\


 Methylamine
Methylamine Pentylamine
Pentylamine Ethanamide
Ethanamide
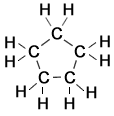
 1,1 dimethyl cyclobutane
1,1 dimethyl cyclobutane 1,3 diethyl cyclohexane
1,3 diethyl cyclohexane