- When multiple bonds form fewer hydrogens are attached to the Carbon atom
- Naming rules are almost the same as with Alkanes.
> The position of the double/ triple bonds ALWAYS has the lowest number and is put in front of the
parent chain.
- double bonds (ALKENES) end in -ene
- triple bonds (ALKYNES) end in -yne
THESE ARE EXAMPLES OF ALKENES AND ALKYNES!
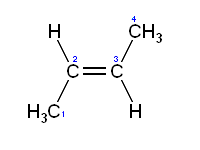
2 Butane:
5 ethyl 2 8 dimethyldecane
1 Butane
EXAMPLES: TRY THESE:
1) Name:
2) Draw: 2,3, Dimethyl-3,4,4 tetra tetrapropyl decane.
3) Draw: 3, 4, 5 Heptyne

No comments:
Post a Comment