CARBOXYLIC ACIDS
- A functional group formed by a doubled bonded O and an OH at the end of a Carbon chain
- Standard rules apply to naming, but change the parent chain ending to -oic acid
- The simplest carboxylic acid is methanoic acid
-
Examples! Name these compounds:
 (butanoic, or butyric acid)
(butanoic, or butyric acid)Now, draw THESE compounds:
Propanoic acid:
Pentanoic acid:
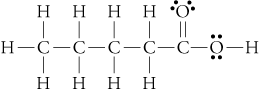
Now heres an extra tricky carboxylic acid that every chemist should know:
Benzenoic Acid:
Great job! Now you're a pro... So lets move on to alcohols.... (dont get TOO excited high school students)
ALCOHOLS
- Alcohols are the functional group that contain OH's as side chains
- There can be OH's on any part of the side chain
- There can be multiple OH's coming off the main chain, and when this happens you use the prefixes di tri tetra etc. (example) methadiol, butatriol etc
- All standard naming rules apply!

Now here are some examples for you try out!: NAME THESE ALCOHOLS:
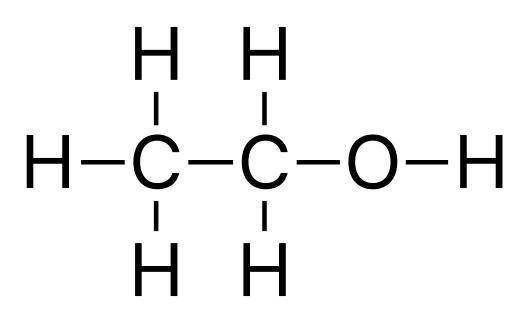 Ethanol
Ethanol Ethanediol
EthanediolDRAW THESE:
propanetriol:

butanediol:
Here again is a super tricky one when benzene becomes an alcohol!
PHENOL:
great work... now lets move on to our final topic ESTERS!
ESTERS!
- Esters first off, are not to be confused with ethers.
- An ester has 2 chains separated by an O, and then one chain right next to the O has a double bonded O on the first C
- Esters are formed when you add carboxylic acids and alcohols together but we'll get into that after we do the basics
- Name the chain without the double bonded O with an -yl ending, and the one with the double O with an -oate ending.
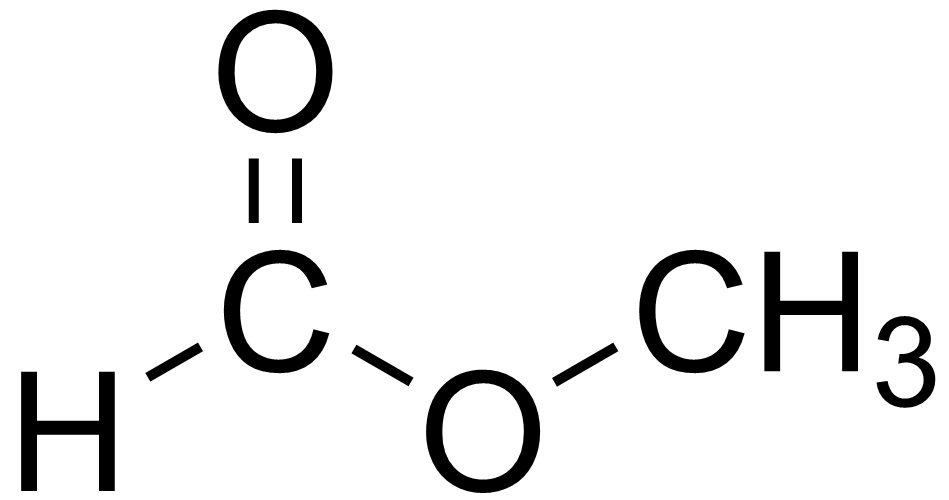
 methyl ethanoate
methyl ethanoate ethyl ethanoate
ethyl ethanoateDraw these!
propyl ethanoate:
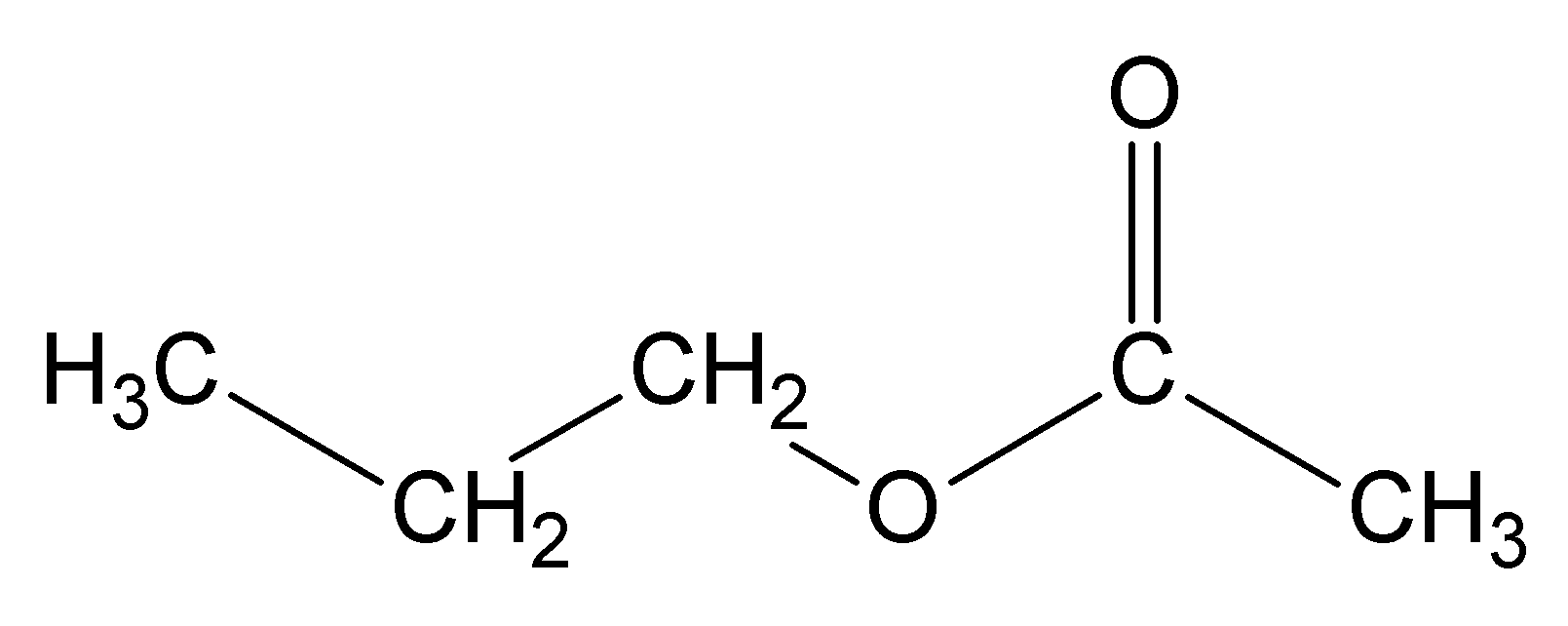
ethyl propanoate:
 \
\and finally, the finale. ESTERFICATION: when a carboxylic acid and an alcohol mix!

Here is a basic explanation. The 2 H's and O form water, and the remainder form the Ester!
Now do these esterfication processes!

GOOD JOB GUYS!
No comments:
Post a Comment