- Elements close to each other on the periodic table display similar characteristics.
- There are SEVEN important periodic trends:
1) Reactivity
2) Ion Charge
3) Melting point
4) Atomic Radius
5) Ionization Energy
6) Electronegativity
7) Density*
REACTIVITY:
- metals and non-metals show different trends.
- the most reactive metal is Francium; the most reactive non-metal is fluorine.
ION CHARGE:
- Elements ion charges depend on their group (column).
MELTING POINT:
- elements in the center of the table of the highest melting point.
- noble gases have the lowest melting points.
- starting from the left to right, melting point increases (until the middle)
> carbonis an exception!
ATOMIC RADIUS:
- radius decreases to the up and the right.
- helium has the smallest atomic radius.
- Francium has the largest atomic radius.
IONIZATION ENERGY:
- ionization energy is the energy needed to completely remove an electron from an atom.
- it increases going up and to the right.
- all noble gases have high ionization energy.
- helium has the highest ionization energy.
- francium has the lowest ionization energy.
- opposite trend from atomic radius.
ELECTRONEGATIVITY:
- refers to how much atoms want to gain elections.
- same trend as ionization energy.
"Chemistry is a class you take in high school or college,where you figure out two plus two is 10, or something."
Thursday, October 28, 2010
Tuesday, October 26, 2010
(DA) Oct. 26, 2010: Isotopes & Atoms
Atomic Number

- Atomic Number: Number of protons

Atomic Number = 22
Symbol = Ti
Atomic Mass = 47.87
Atomic mass - Atomic Number = # neutrons
(p+n) - (p) = (n)
Isotopes - Same atomic number but different mass
FOR EXAMPLE:
Isotope Mass# Atomic# # of Protons #of Neutrons
54Fe 54 26 26 28
56Mn 56 25 25 31
237Np 237 93 93 144
14C 14 6 6 8
Friday, October 22, 2010
(TG) Oct. 21, 2010: Quantum Mechanics
CHEMISTRY NOTES
QUANTUM MECHANICS
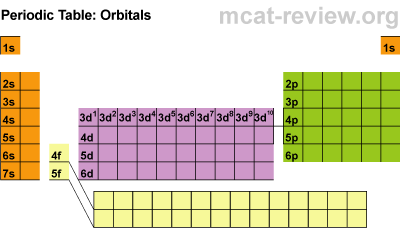
· Different levels of electrons: s, p, d, and f orbitals
· As you increase levels, the orbitals get larger
BOHRS THEORY:
· The electron is a particle that must be in orbital in the atom
QUANTUM THEORY:
· The electron is like a cloud of negative energy or a wave
· Orbitals are areas in 3D spaces where the electrons most probably are
· The energy of the electron is in its vibrational modes – like notes on a guitar string
· Photons are produced when high energy modes change to lower energy modes
S ORBITALS:
· Each orbital holds 2 electrons
(hydrogen and helium)
P ORBITALS
· There are 3 suborbitals
· Each contains 2 electrons
· Total of 6 electrons (3 x 2 = 6)
D ORBITALS
· There are 5 suborbitals
· Each contains 2 electrons
· Total electrons: (2 x 5 = 10)
F ORBITALS
· There are 7 suborbitals
· Each contains 2 electrons
· Total electrons: (7 x 2 = 14)
· Can be made into nuclear bombs
Examples:
1. How many and what type of electrons does the atom calcium have?
1s2 2s2 2p6 3s2 3p6 4s2
Tuesday, October 19, 2010
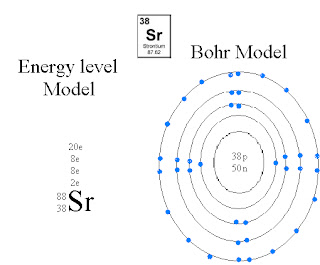
(NR) Oct. 19, 2010: Bohr Diagram
these are examples of the energy level model and the Bohr Model... by Nikko Rey.
Bohr Model
- Atoms are electrically neutral.
- Two different models can be used to describe electron configuration.
> Energy level Model
> Bohr Model
- Electrons occupy shells which are divided into orbitals.
> 2e in the first orbital
> 8e in the second orbital (OCTET)
> 8e in the third orbital (OCTET)
Monday, October 18, 2010
(DA) Oct. 15, 2010: Neils Bohr
Bohr (1920s)

- Rutherford's model was inherently unstable
- Protons and electrons should attract eachother - Matter emits light when it is heated (black body radiation)
- Light travels as photons
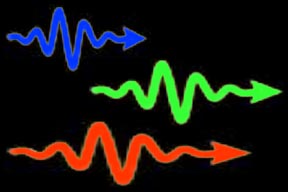
PHOTONS - The energy photons carry depends on their wavelengths
Bohr based his model on the energy (light emitted by different atoms. Each atom has a spectra of light. To explain this emission spectra, Bohr suggested that electrons occupy shells or orbitals

If given enough energy, an atom can move down or up levels of the orbitals.
BOHRS THEORY:
- Electrons exist in ortibals
- When they absorb energy they move to a higher orbital
- As they fall from a higher orbital to a lower one they release energy as a photon of light.
Thursday, October 14, 2010
(TG) October 13, 2010: Atomic Theory
THE ATOMIC THEORY
ARISTOTLE:
- One of the first theories ever created was called the "Four Elements Theory"
- Consisted of: Water, Earth, Wind, and Fire
- Lasted about 2000 years, but is not currently a valid theory because it cannot be proven
- In 300 BC Democritus said atoms were indivisible atoms
- It was the first mention of atoms: atomos
- Was only a conceptual model
- No mention of a nucleus
- Does not explain Nuclear Reactions
- Created the law of the conservation of mass: "in a chemical reaction no matter is destroyed or created"
- Also had a law of definite proportions: (e.g. in a H20 molecule there is always 11% H and 89% O)
- In large proportions atoms have the same ratios as they do in molecules or compounds
- Proved Lavoisiers Laws correct
- Believed atoms are solid, indestructible spheres (e.g. billiard balls)
- This theory provides an explanation for different elements
- Based on the law of conservation of mass (if atoms are not destroyed, mass doesn't change)
- Having a molecule explains the law of of Constant Composition
- 2H2 + O2 = 2H2O
- Raisin Bun model
- solid, positive spheres, with negative particles embedded in them
- first atomic theory to have positives and negatives
- demonstrated the existence of electrons (cathode tube)
- showed that atoms have a positive, dense, center, with electrons outside of it
- resulted in planetary model
- explains why electrons spin around nucleus
- suggests that electrons are made up of mostly open spaces
RAISIN BUN MODEL

RUTHERFORDS MODEL (ELECTRON SPACING)
COMPARING THE MODELS
Tuesday, October 5, 2010
(DA) Oct. 4: Sodium Chloride Lab
Today we started off the class with a simple homework check.
Soon after we were given instructions on how to do our lab by Mr. Doktor (the best teacher in the world, as well as the smartest), and how to use each of the materials.
We split into groups of 3 and 4, and set off to gather our materials and safety clothing and equipment.
As we were in our groups we put our weight paper on the electronic scale and waited for Mr. Doktor to
put the Sodium Chloride (salt) onto the scale.
The Procedure we needed to follow:
1. Gather all the materials and put them on the lab bench
2. Measure 30 mL of distilled water using the graduated cylinder. Transfer this water into a 50 mL beaker
3. Weight 50g of Sodium Chloride.
4. Add Sodium Chloride to the water until it stops dissolving (the solution is saturated) and the first salt crystals begin appearing on the bottom of the beaker.
5. Measure the mass of salt remaining. Record the difference in salt as the amount added to 30 mL of water
6. Repeat steps 2-5 for 50 mL of water, 80 mL of water and 100mL of water.
7. Record all your data in the table below.
8. Create a graph of Mass of \salt vs. Volume of Water (Be sure you include a title, axis, data points, scale and a straight line of best fit).
Electronic Scale:

Graduated Cylinder:

Beaker:

Soon after we were given instructions on how to do our lab by Mr. Doktor (the best teacher in the world, as well as the smartest), and how to use each of the materials.
We split into groups of 3 and 4, and set off to gather our materials and safety clothing and equipment.
As we were in our groups we put our weight paper on the electronic scale and waited for Mr. Doktor to
put the Sodium Chloride (salt) onto the scale.
The Procedure we needed to follow:
1. Gather all the materials and put them on the lab bench
2. Measure 30 mL of distilled water using the graduated cylinder. Transfer this water into a 50 mL beaker
3. Weight 50g of Sodium Chloride.
4. Add Sodium Chloride to the water until it stops dissolving (the solution is saturated) and the first salt crystals begin appearing on the bottom of the beaker.
5. Measure the mass of salt remaining. Record the difference in salt as the amount added to 30 mL of water
6. Repeat steps 2-5 for 50 mL of water, 80 mL of water and 100mL of water.
7. Record all your data in the table below.
8. Create a graph of Mass of \salt vs. Volume of Water (Be sure you include a title, axis, data points, scale and a straight line of best fit).
Electronic Scale:
Graduated Cylinder:
Beaker:
Friday, October 1, 2010
(NR) Sept 30: Density and Graphing
Density
- The density of an object is its mass divided by its volume. It is usually expressed in kg/L, kg/m^3, or g/cm^3.
example:
1) Determine the density of the hot tub that has the mass of 115kg and a volume of 70L.
d = 115 kg
70L
= ____kg/L
(answer: 1.6 kg/L)
Graphing
run 1 run 1 run 2 run 1
= 4 miles/hour = 2 miles/hour = 2 miles/hour = 2 miles/hour
m5) rise = 0
run 1
= 0 mile/hour
AREA:
a = 1/2bh = 1/2(1)(4) d= 1/2bh = 1/2(1)(2)
= 2 = 1
= lw = (10)(1)
b= 1/2bh = 1/2(1)(2) = 10
= 1 = 11
= lw = (4)(1)
= 4 e= lw = (12)(1)
= 5 = 12
c= 1/2bh = 1/2(2)(4)
= 4 TOTAL AREA: a + b + c + d + e
= lw = (2)(6) = 2 + 5 + 16 + 11 + 12
= 12 = 46 miles x hour
= 16
- The density of an object is its mass divided by its volume. It is usually expressed in kg/L, kg/m^3, or g/cm^3.
example:
1) Determine the density of the hot tub that has the mass of 115kg and a volume of 70L.
d = 115 kg
70L
= ____kg/L
(answer: 1.6 kg/L)
Graphing
- All graphs MUST contain 5 important things:
1) Labelled axis
2) Appropriate scale
3) Title
4) Data points
5) Line of best fit line - Three things can be done when working with graphs:
1) Read the graph.
2) Find the slope (RISE/RUN)
3) Find the area under the graph.

run 1 run 1 run 2 run 1
= 4 miles/hour = 2 miles/hour = 2 miles/hour = 2 miles/hour
m5) rise = 0
run 1
= 0 mile/hour
AREA:
a = 1/2bh = 1/2(1)(4) d= 1/2bh = 1/2(1)(2)
= 2 = 1
= lw = (10)(1)
b= 1/2bh = 1/2(1)(2) = 10
= 1 = 11
= lw = (4)(1)
= 4 e= lw = (12)(1)
= 5 = 12
c= 1/2bh = 1/2(2)(4)
= 4 TOTAL AREA: a + b + c + d + e
= lw = (2)(6) = 2 + 5 + 16 + 11 + 12
= 12 = 46 miles x hour
= 16
Subscribe to:
Posts (Atom)